KB4603 (Cure Incorporated Terpolymers)
FKM (Fluorocarbon Rubber or Fluoroelastomer) Raw material
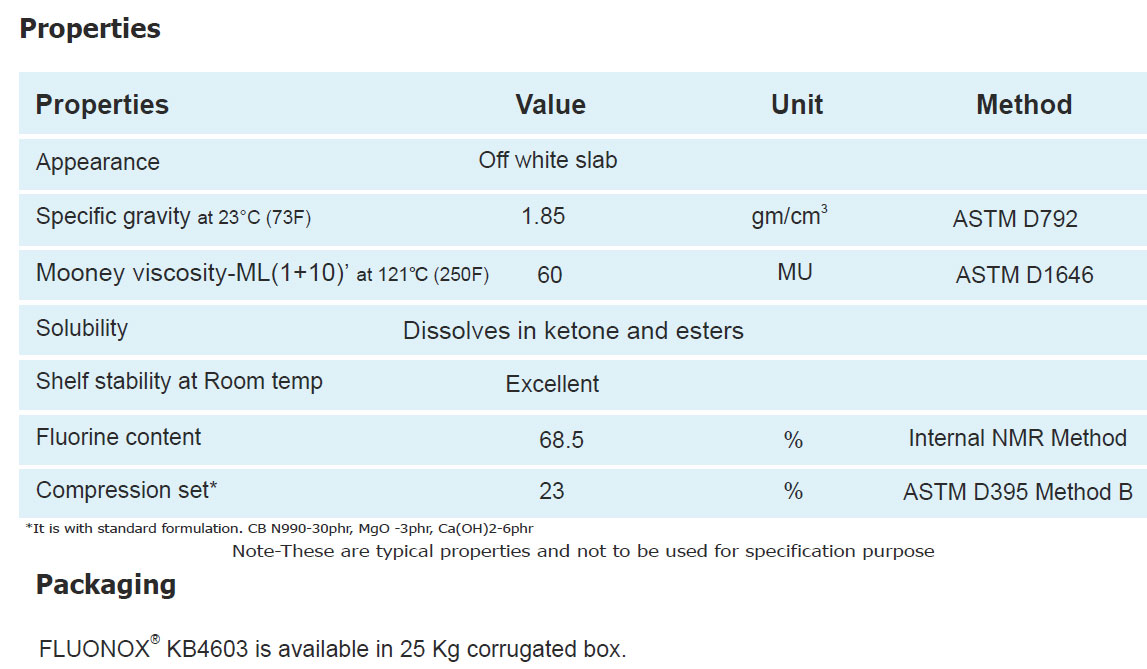
Technical Information
FLUONOX® KB4603 is a fluoroelastomer which consist of HFP , VDF and TFE. FLUONOX® KB4603 is Bisphenol AF cure incorporated fluoroelastomer. It is suitable for compression, Transfer and injection moulding of O-rings . FLUONOX® KB4603 grade is an excellent choice for making O-rings and in oil & gas application.
Product features
* Cure incorporated terpolymer
* Excellent Chemical resistance including oxygenated fuels
* Good choice for O-rings/ gaskets where good compression set is required
Safety and Handling
Handling and processing of fluoroelastomer must be done in ventilated areas to prevent personnel exposure to the fumes liberated during curing or use of cured rubber at high temperature. During the process, some fumes may generate at high temperature which are harmful for human beings. Fumes should not be inhaled, eye and skin contact must be avoided. In case of skin contact flush skin with cold water immediately. In case of eye contact, flush with water immediately and seek medical help. Smoking tobacco or cigarettes should not be allowed in working area. Mixing agents that contain metallic particulate such as powdered aluminum can rapidly decompose at high temperature; therefore do not use metallic particulate as mixing agent.
Fluoroelastomer should be stored away from heat. It should be kept in clean and dry area where it can be protected until it is used. Please read the Material Safety Data Sheet before handling the product.
Disclaimer
FLUONOX is the brand name of Gujarat Fluorochemicals Limited (GFL) used for its brand of fluoroelastomer. FLUONOX can be used in ® ®
applications duly approved by GFL. Customers who plan to use the word FLUONOX® as the trade mark on or relation to their own fluoroelastomer
parts and other products in any style or combination or in any manner whatsoever must contact GFL for prior permission for such use. No
consumer/user of GFL fluoropolymer resin is permitted to claim that their products contain FLUONOX® without prior permission from GFL.
The information provided in the bulletin is furnished at no cost to the recipient and is based on information and technical data that Gujarat
Fluorochemicals Limited believes is correct and sound. Those who choose to use the information must be technically qualified, and do so entirely at
their own cost and risk. The users must determine and insure that their specific conditions of processing present no health or safety hazards. GFL
does not warranty, either expressly or impliedly in respect of use of this information for application of its FLUONOX® branded Fluoroelastomer and
shall bear no liability as a result of any loss or damage caused directly or indirectly due to use of any information provided in this bulletin. Nothing
contained herein can be taken or construed as a grant of license by GFL to operate under or a recommendation to infringe any patents.
Note warning
Do not use any of FLUONOX® Fluoroelastomer in medical devices that are designed for permanent implantation in the human body. For other
medical uses, prior permission of GFL may be sought.
